Aging is a complex process that significantly impacts both individual health and societal structures. Understanding the role of stem cells in aging and disease can provide insights into potential interventions that may enhance our healthspan. This blog delves into the intricate relationship between stem cells and aging and disease, highlighting the challenges and breakthroughs in stem cell research.
Table of Contents
- 🌍 Aging is an Emerging Global Public Health Issue
- 🧬 Stem Cell Superpowers: Self-Renewal & Multilineage Differentiation
- 🦠 Aging of Blood-Forming Stem Cells Leads to Blood & Immune Disorders
- 🧪 The Role of Protein Misfolding in Aging and Stem Cell Function
- 🔬 Reducing Protein Synthesis Alleviates Stress
- 🧪 Aging Stem Cells Sound the Stress Alarm
- ⚔️ A Double-Edged Sword: HSF1 Also Protects Cancer Cells from Stress
- 💪 Keeping Our Aging Stem Cells Fit Increases the Risk of Cancer
- 🧬 Limiting Misfolded Proteins to Keep Stem Cells Fit & Prevent Cancer
- 💪 Keeping Stem Cells Fit to Extend Human Healthspan
- ❓ FAQ
🌍 Aging is an Emerging Global Public Health Issue
Aging is more than just a biological process; it’s a global public health challenge that affects individuals and societies alike. As populations age, we face increased risks of various diseases, including cardiovascular issues, dementia, and more. By the time we reach the age of 28, aging becomes the leading risk factor for disease and death.
According to the World Health Organization, biological aging results from the accumulation of molecular and cellular damage over time. This accumulation leads to a gradual decline in physical and mental capacity, increasing the risk of disease and ultimately mortality. It’s a daunting reality that by 2050, the global population aged over 60 is expected to double, posing significant healthcare challenges.
📊 The Economic Implications of Aging
The aging population is fundamentally reshaping our economy. Individuals aged 55 and older currently represent about 30% of the population but account for 56% of healthcare spending. As people age, their healthcare costs rise dramatically, particularly after age 65, when they will use about half of their lifetime healthcare resources.
- By 2025, the U.S. may face a shortage of healthcare workers, including home health aides and nursing assistants.
- The International Monetary Fund predicts a 10% drop in labor force participation due to the aging population.
- These economic shifts will require innovative solutions to ensure that we can support an aging population effectively.
🧬 Stem Cell Superpowers: Self-Renewal & Multilineage Differentiation
Stem cells are the body’s natural repair system, capable of self-renewal and differentiating into various specialized cell types. Adult stem cells, also known as somatic or tissue stem cells, are present in many tissues, including the brain, muscle, and skin. Their ability to regenerate tissues is crucial for maintaining health.
These stem cells possess two remarkable capabilities:
- Self-Renewal: They can replicate themselves indefinitely, ensuring a continual supply of stem cells.
- Multilineage Differentiation: They can develop into any cell type needed by the tissue, allowing for effective repair and regeneration.
🚨 Aging Causes Stem Cells to Malfunction
As we age, the functionality of our stem cells declines, leading to various degenerative diseases. Aging affects stem cells in three primary ways:
- Loss of Self-Renewal: Stem cells may lose their ability to replicate, leading to a decreased pool of stem cells and impaired tissue regeneration.
- Excess Self-Renewal: Some stem cells may become overly active, leading to the production of too many cells, which can contribute to cancers.
- Skewed Differentiation: The differentiation process may become unbalanced, causing stem cells to produce more of one cell type at the expense of others, leading to tissue dysfunction.
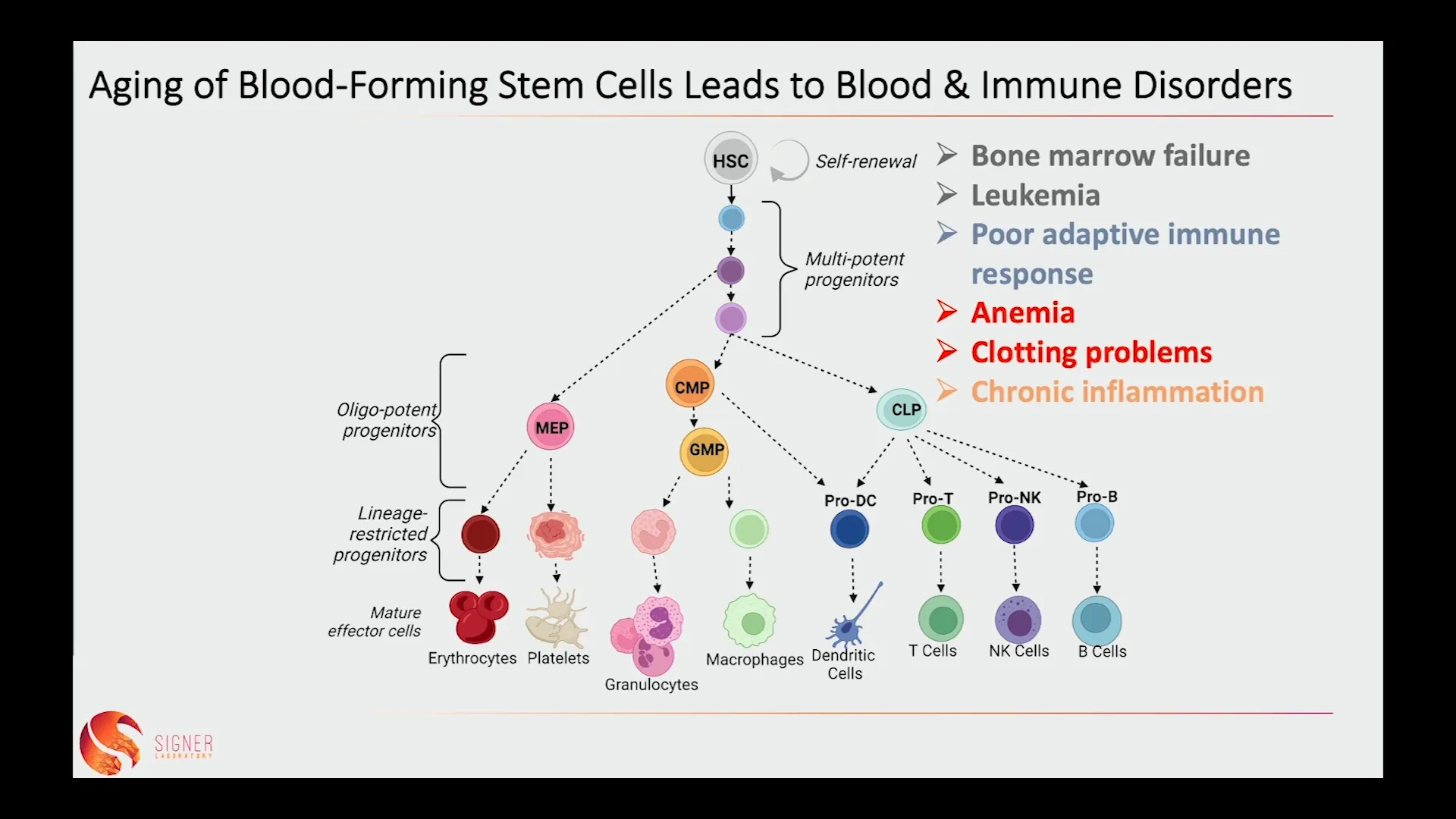
For instance, hematopoietic stem cells, responsible for producing blood and immune cells, can experience these malfunctions, resulting in conditions like bone marrow failure or increased susceptibility to infections.
🦠 Aging of Blood-Forming Stem Cells Leads to Blood & Immune Disorders
The aging of blood-forming stem cells is particularly concerning as it can lead to a host of blood and immune disorders. As these stem cells malfunction, the body struggles to maintain a balanced immune response. This imbalance can result in a decline in adaptive immune cells, making older individuals more prone to infections.
Additionally, the aging process can lead to an increase in inflammatory myeloid cells, contributing to chronic inflammation and further exacerbating age-related diseases. The accumulation of misfolded proteins in stem cells is a key factor in this dysfunction. Stress from various sources accelerates aging and leads to DNA repair defects in stem cell function and aging.
🔬 Understanding and Reversing Stem Cell Aging
To tackle the challenges posed by aging stem cells, we must first understand the underlying mechanisms. One significant factor is the rate of protein synthesis within stem cells. Research indicates that stem cells produce proteins at a slower rate compared to other cell types. This slow production is crucial for maintaining their long-term function and preventing stress accumulation.
Interestingly, studies across various model organisms have shown that reduced protein synthesis correlates with increased lifespan. This finding suggests that promoting low protein synthesis in stem cells could extend their functional lifespan, ultimately improving health span.
🧪 The Role of Protein Misfolding in Aging and Stem Cell Function
Protein misfolding is a significant source of cellular stress. When protein production occurs rapidly, errors increase, leading to misfolded proteins that can be toxic to cells. This toxicity is particularly detrimental to stem cells, which need to maintain pristine protein quality to function effectively.
Our research has shown that aging stem cells accumulate misfolded proteins, which can impair their self-renewal capabilities and lead to tissue dysfunction. A critical gene in this process is heat shock factor 1 (HSF1), which helps stem cells manage stress by activating protective pathways. However, when HSF1 is overly activated, it can also promote cancer cell growth.
⚖️ The Double-Edged Sword of Stress Response
The challenge lies in balancing the benefits of stress response mechanisms while preventing the risk of cancer. Our goal is to limit the production of misfolded proteins to keep stem cells fit without activating pathways that could lead to cancer development.
By focusing on minimizing protein synthesis errors, we aim to enhance stem cell longevity and functionality. Recent advancements have allowed us to introduce specific mutations in DNA that improve protein quality, potentially slowing the aging process of stem cells.
In conclusion, the intricate relationship between stem cells and aging is critical for understanding how to enhance health span and combat age-related diseases. By continuing to explore these mechanisms, we can develop strategies to maintain stem cell function and promote healthier aging.
🔬 Reducing Protein Synthesis Alleviates Stress
As we delve deeper into the molecular biology of stem cells and aging, one of the most compelling findings is the role of protein synthesis in stem cell function. Research indicates that stem cells operate optimally with a slower rate of protein synthesis. This deliberate reduction is not just a quirk; it’s a protective mechanism against the accumulation of misfolded proteins, which can lead to cellular stress.
High rates of protein synthesis can overwhelm the cellular machinery, leading to an increase in misfolded proteins. Such proteins can accumulate and cause significant stress within the cell, ultimately impairing stem cell function. By maintaining a lower rate of protein synthesis, stem cells can reduce the risk of these detrimental effects, thereby promoting their longevity and functionality.
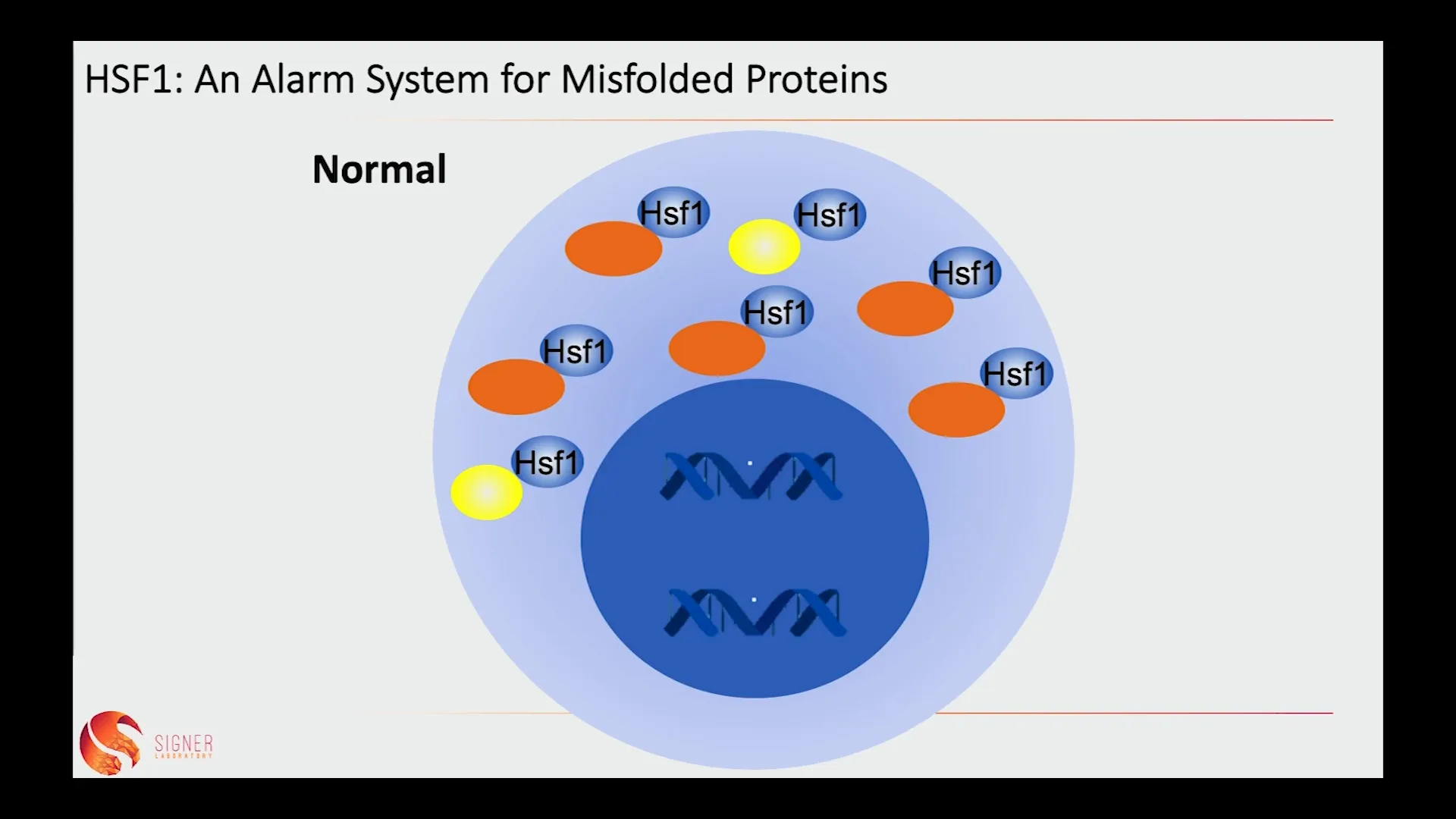
🧬 HSF1: An Alarm System for Misfolded Proteins
Heat shock factor 1 (HSF1) plays a pivotal role in the stress response of stem cells. Under normal conditions, HSF1 remains in the cytoplasm, bound to chaperone proteins that assist in proper protein folding. However, when misfolded proteins accumulate due to stress, HSF1 is released and translocates to the nucleus. This movement triggers a cascade of protective responses aimed at restoring cellular homeostasis.
Interestingly, while HSF1 is crucial for maintaining stem cell health, it also presents a potential risk. In the context of aging, the activation of HSF1 can lead to an increase in the proliferation of cancer cells. This dual role of HSF1 underscores the complexity of stem cell biology and the delicate balance that must be maintained to prevent both aging and disease.
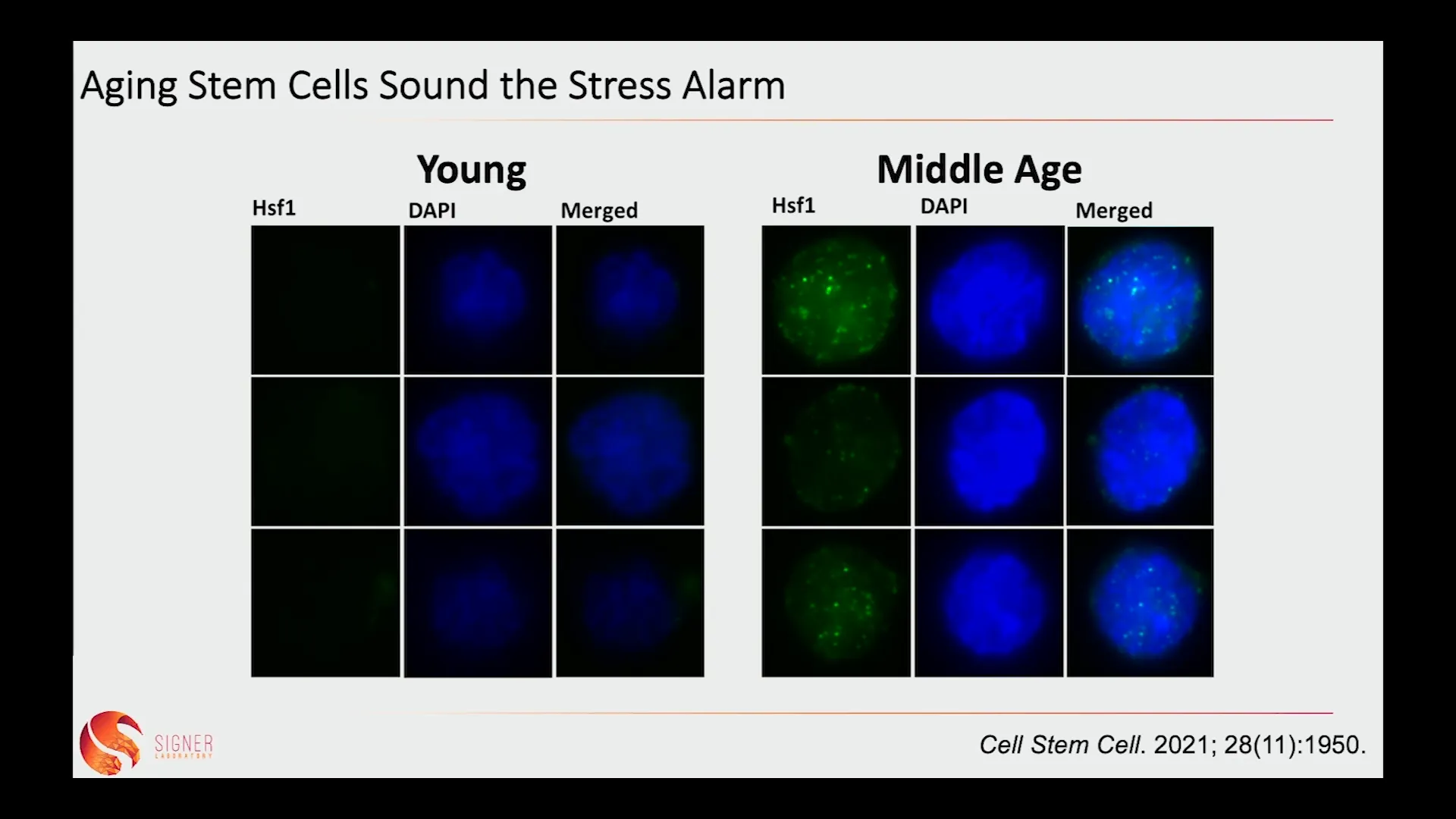
🧪 Aging Stem Cells Sound the Stress Alarm
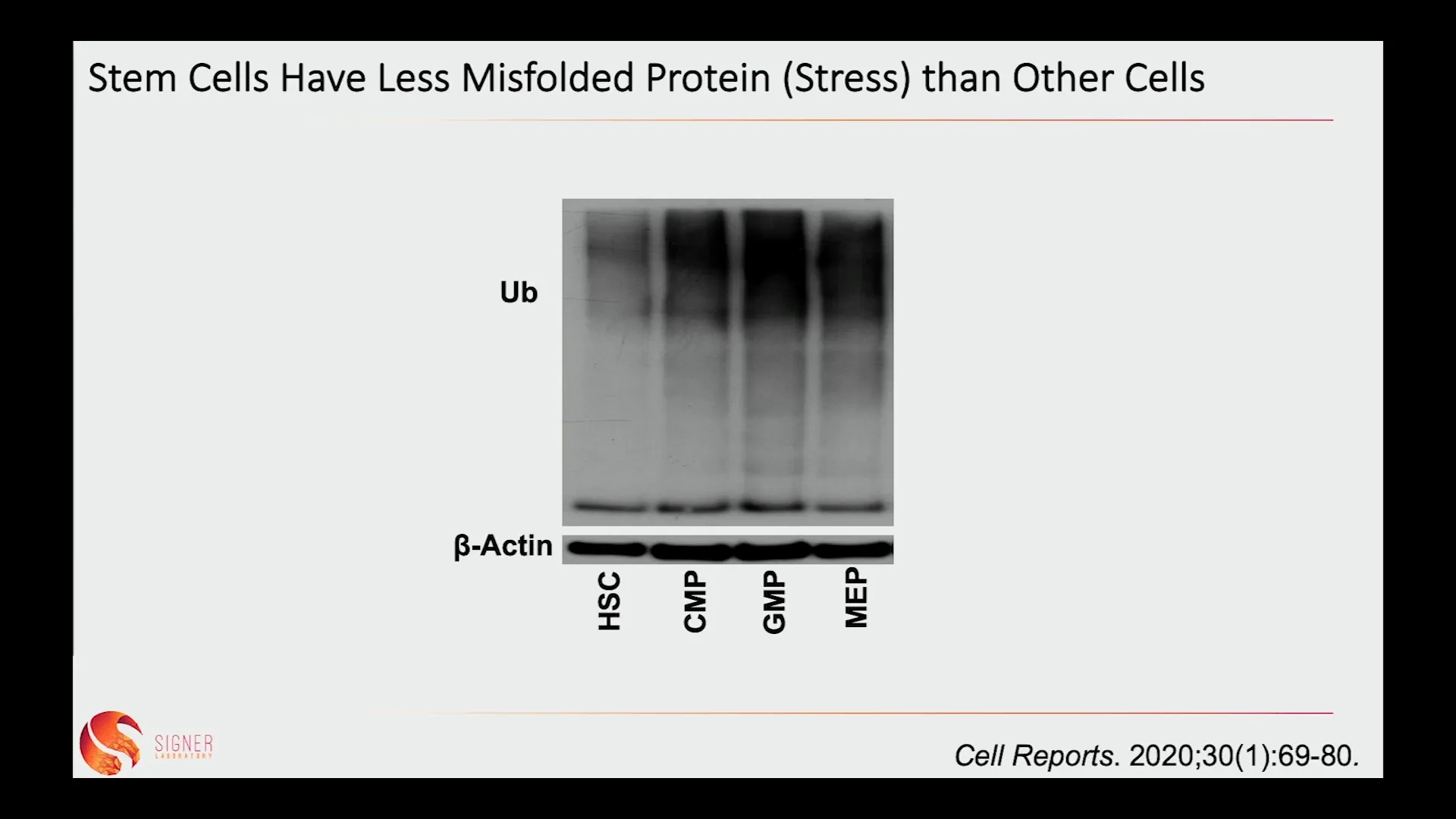
As stem cells age, they begin to exhibit signs of stress accumulation. One of the most alarming observations is that aging stem cells start to accumulate aggregated proteins, which can significantly impair their function. This accumulation is a direct consequence of the inefficiency of the cellular machinery to manage protein folding as it ages.
Our laboratory has observed that aging stem cells show a 20-30% increase in aggregated proteins compared to their younger counterparts. This increase in aggregated proteins is detrimental, as it can lead to impaired self-renewal and an inability to differentiate properly, ultimately contributing to tissue dysfunction.
⚠️ Aging Stem Cells Accumulate Aggregated Proteins
The accumulation of aggregated proteins in aging stem cells has profound implications. Not only does it affect the stem cells’ ability to regenerate tissues, but it also contributes to the overall aging process. The presence of these aggregates signals a breakdown in the stem cells’ ability to maintain homeostasis, further exacerbating the aging phenotype.
In a healthy system, stem cells efficiently manage protein quality. However, as they age, the balance tips, leading to an increase in stress and dysfunction. This phenomenon highlights the critical need for interventions aimed at maintaining stem cell health as we age.
⚔️ A Double-Edged Sword: HSF1 Also Protects Cancer Cells from Stress
While HSF1 is essential for maintaining the health of aging stem cells, it also has a darker side. The same mechanisms that protect stem cells can inadvertently aid cancer cells in their survival and proliferation. This is particularly evident in conditions such as acute myeloid leukemia, where HSF1 activation supports the growth of cancer cells.
Research shows that when HSF1 is activated in leukemia cells, it enhances their ability to cope with stress, promoting tumor growth. This duality presents a significant challenge: how can we harness the protective benefits of HSF1 for stem cells while minimizing its potential to support cancer development?
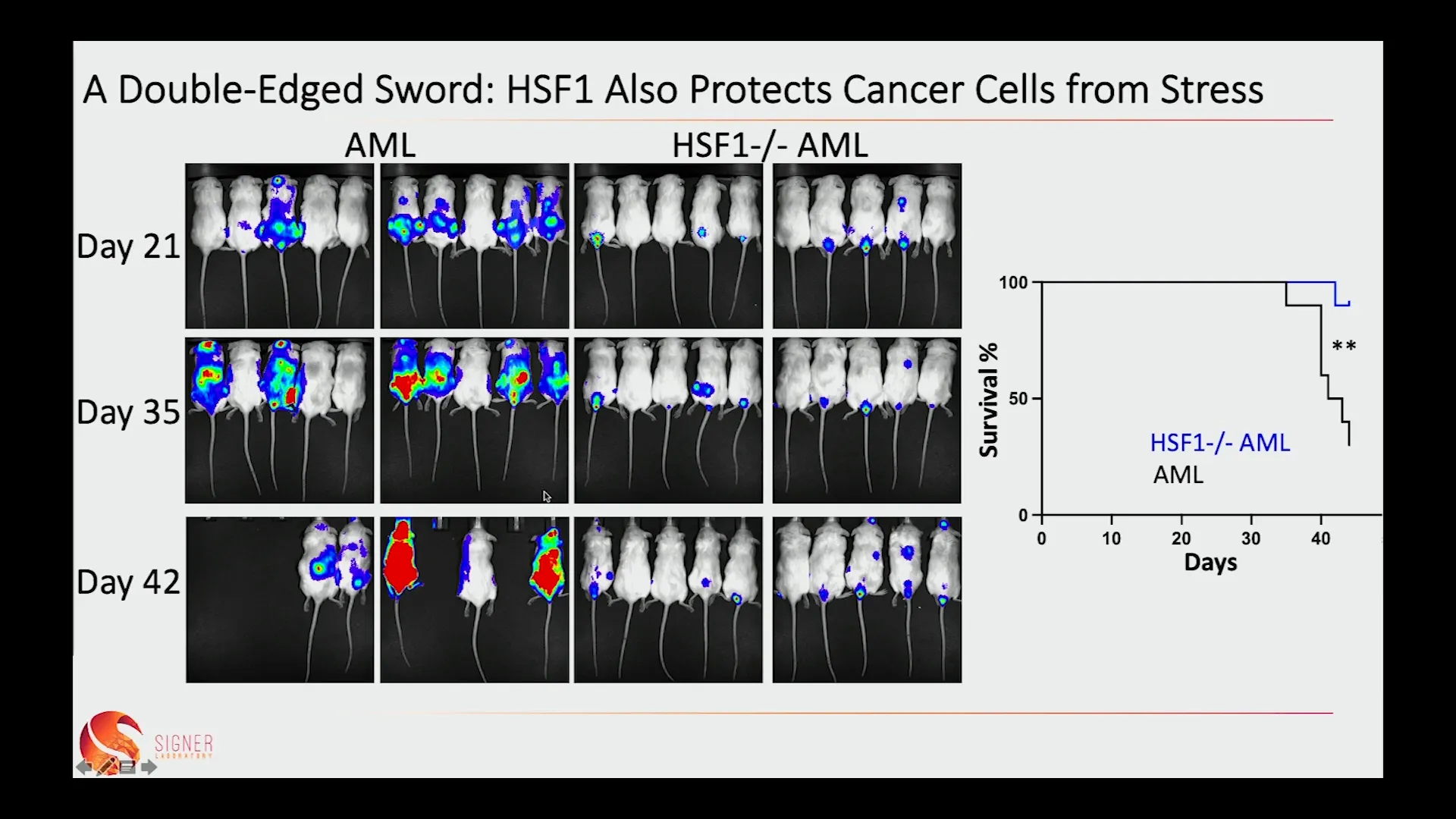
💪 Keeping Our Aging Stem Cells Fit Increases the Risk of Cancer
As we explore the relationship between stem cell function and aging, it becomes clear that maintaining stem cell fitness is essential for both regenerative capacity and overall health. However, there is a fine line between keeping stem cells fit and inadvertently increasing the risk of cancer. The strategies we employ to enhance stem cell health must be carefully balanced to avoid tipping the scales toward malignancy.
To prevent the accumulation of misfolded proteins, we must focus on minimizing the errors in protein synthesis. By introducing specific mutations that enhance the accuracy of protein production, we can potentially slow down the aging process of stem cells while mitigating the risk of cancer. This approach shows promise in extending the health span, allowing for better tissue regeneration without the adverse effects associated with cancer.
🔍 Understanding and Reversing Stem Cell Aging
Our understanding of stem cells and aging is rapidly evolving, and with it, the potential for therapeutic interventions. By targeting the molecular mechanisms that underlie stem cell aging, we can develop strategies to reverse or at least slow down the aging process. This involves not only managing protein synthesis but also enhancing the cellular machinery responsible for maintaining protein quality.
As we continue to investigate the molecular biology of stem cells, we are optimistic about the future. The potential to reverse stem cell aging could lead to breakthroughs in regenerative medicine, offering new hope for age-related diseases and enhancing health span.

In summary, the intricate relationship between stem cells and aging reveals not only the challenges we face but also the opportunities for intervention. By understanding the molecular biology of stem cells and their role in aging and disease, we can develop targeted therapies that promote healthier aging and improve overall quality of life.
🧬 Limiting Misfolded Proteins to Keep Stem Cells Fit & Prevent Cancer
As we explore the intricate relationship between aging and stem cells, one of the critical aspects is the accumulation of misfolded proteins. This accumulation not only hinders stem cell functionality but also increases the risk of cancer. The aging process leads to a deterioration in the cellular mechanisms responsible for maintaining protein quality, which can have dire consequences for stem cell health.
Research has shown that aging stem cells face significant challenges in managing misfolded proteins. When proteins misfold, they can aggregate and become toxic, leading to cellular stress that impairs stem cell function. This situation creates a vicious cycle where stem cells become less effective at replenishing tissues, thereby contributing to age-related diseases.
🔍 Strategy: Make Protein Synthesis Less Error-Prone
To combat the detrimental effects of aging on stem cells, we need to focus on improving the accuracy of protein synthesis. The goal is to reduce the errors in protein production that lead to misfolded proteins. Innovations in genetic engineering have made it possible to introduce specific mutations that enhance the fidelity of protein synthesis.
By optimizing the machinery responsible for protein production, we can potentially minimize the stress on stem cells and extend their functional lifespan. Studies indicate that a slight reduction in the rate of protein synthesis can lead to a significant decrease in the accumulation of misfolded proteins, ultimately promoting healthier aging.
💪 Keeping Stem Cells Fit to Extend Human Healthspan
Aging is a primary risk factor for a myriad of diseases, including cancer, cardiovascular diseases, and neurodegenerative disorders. Understanding the mechanisms behind stem cell aging is crucial for extending the human healthspan. Healthspan refers to the period in which an individual remains healthy and free from chronic diseases.
To achieve this, we must develop interventions that target the underlying causes of stem cell dysfunction. This includes addressing the DNA repair defects in stem cell function and aging. As stem cells age, their ability to repair DNA diminishes, leading to further cellular damage and dysfunction.
By focusing on enhancing DNA repair mechanisms, we can improve stem cell resilience and functionality, paving the way for healthier aging. This approach not only benefits the individual but also alleviates the societal burden posed by aging populations.
📚 Understanding and Reversing Stem Cell Aging
Research into understanding and reversing stem cell aging is rapidly advancing. The molecular biology of stem cells reveals that the key to longevity may lie in the intricate balance of protein synthesis, DNA repair, and cellular stress responses. By targeting these elements, we can devise strategies to rejuvenate aging stem cells.
One promising avenue is the exploration of compounds that enhance stem cell function by reducing oxidative stress and improving DNA repair capabilities. This could lead to significant breakthroughs in regenerative medicine and therapies aimed at age-related diseases.
Ultimately, the goal is to translate these scientific findings into practical applications that promote healthy aging and extend the healthspan of individuals. By fostering a better understanding of stem cells and aging, we can unlock the potential for innovative treatments that improve quality of life for aging populations.

❓ FAQ
What role do stem cells play in aging?
Stem cells are essential for tissue regeneration and repair. As we age, their functionality declines, leading to various age-related diseases. Understanding this decline is crucial for developing interventions that can enhance stem cell performance and promote healthy aging.
How do DNA repair defects affect stem cell function?
DNA repair defects in stem cells can lead to accumulated damage, impairing their ability to self-renew and differentiate properly. This dysfunction is a significant contributor to aging and the onset of age-related diseases.
Can stem cells be rejuvenated?
Yes, research is ongoing to explore methods for rejuvenating aging stem cells. Strategies include optimizing protein synthesis, enhancing DNA repair mechanisms, and reducing oxidative stress, all of which can improve stem cell function and longevity.
What is the relationship between stem cells and cancer?
While stem cells are vital for regeneration, their dysfunction can also lead to cancer. As stem cells age, the risk of accumulating mutations increases, which can result in uncontrolled cell growth and tumor formation. Balancing stem cell health is essential to mitigate this risk.
In summary, the intersection of aging and stem cells presents both challenges and opportunities. By understanding and addressing the factors that influence stem cell function, we can make significant strides in promoting healthier aging and improving the quality of life for individuals across the globe.
Stem Cell Therapy Technology
Reboot Your Health with Stem Cell Technology – Try LifeWave X39! Experience the transformative benefits of stem cell activation with LifeWave X39. Commit to a 3-month trial and feel the difference in your energy, healing, and vitality. * Wholesale Trial Price – $99/month for 3 months ($297 total), plus a one-time $20 fee for wholesale access. * Postage Costs – Additional shipping charges apply. * Retail Option – $150/month for 3 months ($450 total). If you’re not amazed after 3 months, we’ll offer a refund! Ready to start? Email us below with the subject “TechTest” to begin your wellness journey.

